Section 5.2: Introduction to laser machining of polymers
Content:
Fundamental issues considered in laser machining of polymers
Polymers consist of long chain molecules with repeating groups that are largely covalently bonded. Common elements within the chain backbone include C, O, N, and Si. An example of a common polymer with a simple structure, polyethylene, is shown in Figure (1). The bonds within the backbone are all covalent, so the molecular chains are extremely strong. Chains are usually bonded to each other, however, by means of comparatively weak secondary bonds. This means that it is generally easy for the chains to slide by one another when forces are applied and the strength is thus relatively low. Note that in contrast to the monomer, PE polymer chain is saturated, so there are no additional sites for primary bond formation. Thus the only mechanism that remains for bond formation between PE chains is secondary bond formation. Linear polymers that form melts upon heating, such as PE, are called thermoplastic polymers. The structure of rubber is fundamentally different from that of the thermoplastic polymers, the Figure (2) show that polymer chains contain an unsaturated bond. The existence of this double bond within the macromolecule permits the formation of additional primary bonds between chains. The primary bonds between rubber chains formed by the opening of the unsaturated double bonds are known as crosslinks. As the crosslink density increases, the individual chains lose their identity and the structure resembles a three-demensional network of primary bonds. This 3-D structure is characteristic of many polymers that do not form a melt, or thermoset polymers.
Fig.1 The structure of polyethylene, PE: (a) the basic building block for PE is the C2H4 monomer; (b)the double bond in the monomer is opened so that (c) many monomers can be linked together to form PE polymer chain; (d) since the polymer chains are saturated, the only type of bonds that can form PE chains is the secondary bonds. (After James P. Shaffer, et al., 1995)
Fig. 2 The structure of crosslinked rubber. The existence of double bonds along the length of the polymer chains shown in part(a)permits the formation of crosslinks between chains, as shown in part (b). (After James P. Shaffer, et al., 1995)
In addition, many polymers tend to soften at moderate temperatures, so they are not generally useful for high-temperature applications. Polymers, however, have properties that make them attractive in many applications. Since they contain common elements and are relatively easy to synthesize, or exist in nature, they can be inexpensive. They have a low density (in part because of the light elements from which they are constituted) and are easily formed into complex shapes. They have thus replaced metals for molded parts in automobiles and aircraft applications, especially where the load-bearing requirements are modest. Because of these properties, as well as their chemical inertness, they are used as beverage containers and as piping in plumbing applications. Like metals and ceramics, their properties can be modified by compositional changes and by processing. For example, substitution of a benzene ring for one in four hydrogen atoms converts polyethylene to polystyrene. Polyethylene is pliable and is used for applications such as "squeeze bottles." In polystyrene, the comparatively large benzene side group restricts the motion of the long chain molecules and makes the structure more rigid. If the benzene group in polystyrene is replaced with a Cl atom (intermediate in size between H and the benzene ring), polyvinylchloride is produced. The Cl atom will restrict the chain mobility more than an H atom but less than a benzene ring. A leathery material is produced with somewhat intermediate properties between polyethylene and polystyrene. These three polymers illustrate the fundamental principle, applicable to a materials, of the relationship between material structure and properties. Some current and potential applications for polymers include: The development of biodegradable polymers offers the potential for minirnizing the negative impact on our environment that results from the tremendous amount of waste our society generates. Advances in liquid-crystal-polymers technology may permit development of lightweight structural materials. Electrically conducting polymers may be able to replace traditional metal wires in weight-critical applications such as electrical cables in aerospace vehicles.
Polymer Ablation Laser machining of polymers involves ablation, in which the matter is ejected in the form of species such as atoms, molecules, ions, and clusters because of the interaction of an intense laser pulse with the polymer material. The macroscopic effects of ablation include plasma, acoustic shocks, and cratering of the surface. Nearly all organic polymers show moderate to intense absorption in the ultraviolet region. These absorptions are usually ascribed to electronic transitions from a ground singlet to the first excited singlet states. The unique features in the UV laser ablation of polymers are encountered only in those wavelength regions in which such electronic absorptions exist.
The ablation of the surface of a polymer by a UV laser pulse is a function of the energy deposited in the solid in unit time. If a typical UV pulse has a full width at half-maximum (FWHM) of 20 ns and an energy of 450 mJ and the size of the beam at the polymer surface is 1.5 cm2, the fluence at the surface will be 300 MJ/CM2 and the power density will be 1.5 x 101 W/cm2. When this pulse strikes the surface a loud audible report will be heard and, depending upon the wavelength, 0.01-0.1 micron of the material would have been etched away with a geometry that is defined by the light beam. If this experiment is performed in air, a bright plume will be ejected from the surface and will extend to a few millimeters. Typically, UV laser ablation is carried out with a succession of pulses. R.Srinivasan and Bodil (1984) Braren have shown that the depth etched is a linear function of the number of pulses but note that there is a very long extrapolation between the origin (zero pulses) and the first data point. One should keep in mind that it is the power density (power/unit area) that is important and discussions based on fluence are acceptable only so long as the pulse width is constant. There is always a threshold value for the fluence for the onset of etching and it is difficult to pinpoint this exactly because the etch curve approaches the abscissa asymptotically. The slopes of the lines give an average value for the etch depth/pulse at that wavelength and fluence for that material. These values are reproducible within the uncertainties in the measurement of the fluence of the laser and the depth of the etching.
A pictorial representation of the interaction of a laser pulse with a polymer surface is shown in Figure 3. As shown in Figure 3(a), the stream of photons from a single laser pulse falls on the polymer and is absorbed in a depth that can he as little as a fraction of a micron for intense absorbers to many tens of microns for weakly absorbing polymers. Obviously, weak absorption and strong absorption refer to specific wavelengths so that the same polymer can absorb weakly at one laser wavelength and strongly at another. Figure 3(b) shows that within the absorption depth, there are numerous bond breaks. In Figure 3(c), the fragments are shown to be ejected from the surface, leaving an etched pit behind.
Fig. 3 Schematic impact of laser pulse on polymer surface. (After R.Srinivasan and Bodil, 1988)
A knowledge of the timing of the ablation process is fundamental to an understanding of the chemical physics of the phenomenon. Early attempts by Koren and Yeh, Davis and co-workers, were based on a spectroscopic investigation of the light emission that accompanies the impact of a UV laser pulse on a polymer surface.They pointed out that "it is possible that the photo dissociation processes responsible for creating the emission in the plume are separate and subsequent to the breaking of the polymeric bonds which cause ablation". They showed that the polymeric structure could begin to ablate on a time scale that is even shorter than the width of a pulse from the laser beam.
Dyer and Srinivasan (1996) measured the time profile of the ablation process in PNFNU and other polymers. They found that when the electrical circuitry for the detection of the transducer signal was taken into account, the overall display system had a rise time that was estimated to be <5 ns. There is a time delay in the detection of the stress wave that is caused by its passage through the PMMA layer before it strikes the surface of the PVDF film. The local heating produced by a laser pulse at a fluence that is well below the threshold value at that pulse width causes a stress wave that is sinusoidal in character. The compression wave caused by the heating is rapidly followed by a rarefaction wave caused by the cooling. As the fluence is increased, the onset of ablation is marked by a transition to an initially structured and then a relatively smooth compressive signal. When the fluence is sufficient to initiate strong ablation, the temporal width of the stress wave is about equal to that of the laser pulse and the point of initiation of both signals is nearly coincident. Partucular note should be taken of the large magnitudes of the stress pulses that are generated in the ablation of both polymers by UV laser pulses. Figure 4 is a plot of the peak amplitude of the stress wave as a function of the fluence for the ablation of a polyimide film.The depth of the polymer that is etched is less than 1micron per pulse at 193-nm wavelength. The ablated material is known to be ejected at 2-5 times the velocity of sound in air (vide infra). It is interesting that this stress pulse extends even below the fluence at which significant etching is observed. Radicals and some ions are emitted at high velocity (which can be as large as 5E5cm/s) at fluences at which the etch depth per pulse is below the limit for detection (about 500 A even after 1000 pulses).
Fig. 4 Peak amplitude of the stress wave as a function of fluence of polymide film irradiated with the 308nm and 193nm lasers. (Dyer and Srinivasan, 1996)
The general features of UV laser ablation of polymers are summarized as follows:
• Polymer ablation takes place within 10 to 100 nanoseconds.
• The threshold energy fluence, defined as the fluence at which the etch depth is 0.05 um per pulse, is low for polymers (typically in the range 10 to 100 MJ/CMI).
• For fluences near or below the threshold, the etch depth follows Beer-Lambert's law (photo-chemical, linear absorption). For fluences above the threshold, thermal effects contribute to the etch depth. In addition, the longer the wavelength, the stronger are the thermal effects.
• Wavelength affects absorption and threshold fluence. The etch depth per pulse (lower absorption coefficient) is larger for a weaker absorber than for a stronger absorber.
• The formation and expansion of the plasma plume during the laser pulse characterize the rapid etching process. The etch depth per pulse increases with energy fluence until the phenomenon of saturation is reached. "Saturation" is a mechanism involving the blocking of the trailing part of the laser pulse by both the plume and the excited polymer species generated by the leading part of the pulse. This occurs only at high energy densities and prevents additional material removal.
• The relaxation rate, the period of time in which the excited state of the polymer endures, affects the absorption of the laser light by the material. If the relaxation rate is too slow compared to the excitation rate, the bleaching or blocking effect occurs and reduces the absorption of laser energy.
• Ablation is accompanied by an acoustic signal that decreases with increasing laser wavelength.
• Ablation creates numerous products, including monomers, low-molecular-weight products, and fragments normal to the surface. The velocities of ablation products are high, in the range 101 to 101 m/s. The velocity distribution of ejected material is not dependent on the energy fluence.
• Ablation takes place in the temperature range 400 to 800C.
• The small absorption depth coupled with short laser pulses and low thermal conductivity of polymers restricts the extent of heat transfer, leading to precise material removal and a small heat-affected zone.
Micromachining of polymers is an important field that has both immediate and future applications in diverse fields such as medicine, microelectromechanical systems, and photonics. For example, the feature size of integrated circuit chips has been reduced nearly 20 times over the past three decades. There is growing interest in the precise fabrication of micro- and nanostructures such as motors, optics, sensors, fluid control devices, actuators, miniature valves, pacemakers, implants, and catheters. The traditional mechanical approaches of cutting, drilling, and shaping materials are no longer satisfactory for fabricating micron-scale structures. Instead, beam techniques based on photons, electrons, and ions are used to produce high-resolution structures. Lasers have been proven as effective tools in micromachining. Lasers have been used to solve fine machining problems in numerous fields, including for medical devices, telecommunication, microelectronics, fiber optics, data storage, instrumentation, and micro-optics. Compared with other technologies for micromachining applications, lasers are suitable for those applications that demand more precision, speed, and "direct-write" capability. Lasers can also work on most materials and are environmentally friendly. In competing against nonlaser techniques, the growth opportunities for laser micromachining will occur in the these areas:
Microstructuring/microdrilling is a process of patterning or structuring electronic devices. Wet and chemical etching processes are not fully satisfactory because they involve several steps, are useful for only silicon and metals, and are prone to environmental disposal problems. Electrical discharge machining (EDM) is suitable for conductive materials with limited aspect ratios and features as small as 10 pm. Focused ion beam is the currently available precision technology capable of reaching the nanometer range.
Mold-insert fabrication is the process of creating a master from which high-volume plastic parts are produced. In terms of speed and convenience for rapid prototyping, the laser is superior to micro EDM, plasma etch, and mechanical machining.
Cutting/excising of silicon wafers and printed circuit boards is performed to produce finished parts. Lasers surpass diamond sawing in meeting the demands for smaller kerf and tight radius pattern. Additionally, the laser is preferred to other processes that have some serious environmental constraints, such as high-pressure water, ultrasonic, and wet chemical.
Selective material removal involves removing a coating or blind-hole drilling with good depth control. Lasers have precise spatial control, reach inaccessible areas, and produce controlled depths with a minimal heat-affected zone (HAZ).
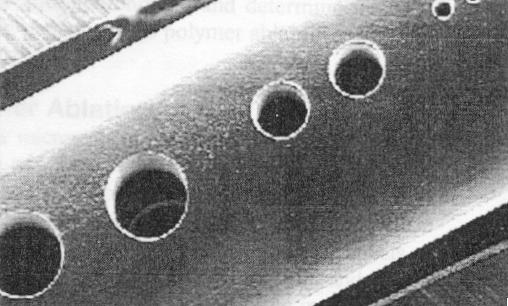
(a) Cylindrical holes (25um,100um,200um)in catheter; (b) Taped holes (25um) in polyamide
Figure 5 Laser Micromachined Features (Illy, Elizabeth K, et. al., 1997)
Ultraviolet (UV) lasers are widely used for micromachining applications. The most popular and commercial UV lasers include excimer, argon-ion, tripled and quadrupled ND:YAG, fluorine, helium-cadmium, metal vapor, and nitrogen. Among these, excimer lasers (193 to 351 nm) are the best choices, but ND:YAG lasers may be considered if they are Q-switched and frequency-tripled to produce 266 nm. Excimer lasers are extensively used for photoablation, chemical etching, lithography, and surface cleaning. The benefits of excimer lasers are attributed to their short wavelengths (193 to 351 nm), high energy per pulse, and nanosecond (ns) pulse widths. The small wave-lengths allow strong interactions of the beam with a variety of workpiece materials. Excimer lasers are best utilized when feature sizes are small and densely packed (Figure 5) and material thickness is minimal. It is now commonplace to create via holes as small as 10 micron in polyimide dielectrics for electronic packaging.